We offer patients what no one else can:
MULTIPLE FAT INJECTIONS FROM A SINGLE HARVEST

Our Purpose
At Tissue and Cell Technologies (T&CT), our mission is radically change how doctors practice medicine.
We are focused on several sectors, all with strong clinical drivers, unmet clinical needs, and potential for high impact on clinical economics and patient care.
Our product, the Adiposet® System, is aimed at unlocking the full potential of regenerative medicine, cell-based therapies, and personalized medicine.
With no rival technology for the high viability and long-term storage of large volumes of human tissue available or visible on the horizon, T&CT’s Adiposet storage technology fills a big gap in the US, Europe, and beyond.

Our Purpose
At Tissue & Cell Technologies (T&CT), our mission is to radically change how doctors practice medicine.
Our technology platform, the ADIPOSET® System, is aimed at unlocking the full potential of aesthetic and regenerative medicine, cell-based therapies, and personalized medicine.
We are focused on several sectors, all with strong clinical drivers, unmet clinical needs, and potential for high impact on clinical economics and patient care.
With no rival technology or company in the long-term storage of large volumes of human adipose tissue, T&CT’s ADIPOSET® storage technology fills a big gap in the US, Europe, and beyond.
Fat Banking & Tissue Storage
Key Clinical Sectors

Aesthetic
Plastic surgery including breast augmentation, BBL, bodysculpting, and skin rejuvenation
Read More
How We Got Here
(T&CT) was founded in 2011 with a pioneering vision to develop a platform for long-term cryo-storage of large volumes of human tissue and cells. Our technology, which maintains cell vigor, viability, structure, and full functionality, is at the forefront of advancements in reconstructive surgery and regenerative medicine.
Fat banking has been referred to as a “Holy Grail” and one of the ten most important advances in plastic surgery in the last 20 years.
Our Proprietary Technology
The ADIPOSET System®
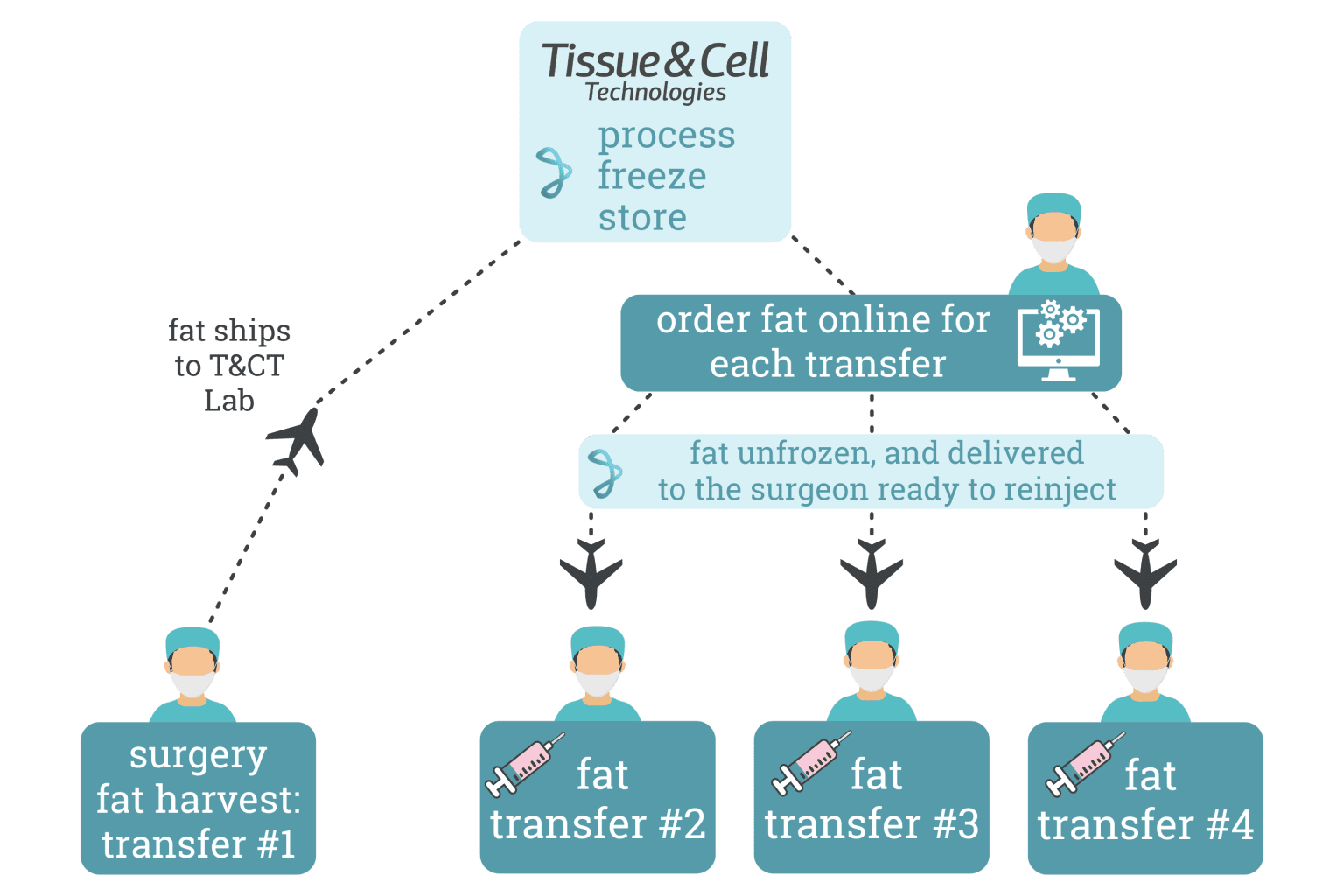
The ADIPOSET® Platform is a versatile tool that stands to transcend traditional medical boundaries. Besides applications in aesthetic and reconstructive surgery, its has potential for playing a key role in emerging fields like regenerative medicine, cell therapies, and personalized medicine.
Where regulation allows, we aim to offer incredible potential for cell extractions, enhancements, manipulation, and expansion, opening up a world of possibilities. Our technology also offers possibilities to store other tissue and cell types, including muscle, heart muscle, skin, cartilage and nerves opening the path to full organ regeneration, muscle damage reversal, and rejuvenative therapies.
A Robust Clinical History
T&CT continued its R&D with the development of Regenerys™, a small volume fat grafting/injection product as an ‘all-natural’ alternative to cosmetic facial fillers. Partnering with a team of gifted specialists in the field of Plastic Surgery, T&CT’s Regenerys™ product is now exclusively available in the U.K. in London’s top plastic surgery clinics, and is planned for broader roll-out in 2021.
T&CT has also responded to calls by a number of reconstructive surgeons and plastic surgeons to make the service available in overseas markets with the planned launch of fully staffed local operations in Dubai and Europe. Both Adiposet™ and Regenerys™ will then be available across all 3 regions, backed by leading clinics and surgeons. Our advanced proprietary skin grafting products, MySkin™ and CryoSkin™ will also be launched into the Middle East.


